This is a short history of modern pandemics, starting with the Great Influenza of 1918, and ending with the modern coronaviruses, SARs, MERs and COVID-19. Most of the last part of this article contains a short history of recent coronaviruses and vaccine development by one of the leading vaccine experts in the world. The second-to-last paragraph describes the strides made in developing a vaccine to counteract COVID-19.
The best source for the 1918 pandemic is John M. Barry’s The Great Influenza: The Epic Story of the Deadliest Pandemic in History of 1918 (Penguin, 2005). This book is now ranked #51 on amazon.com, despite being released more than 15 years ago.
Barry was born in Providence and graduated from Brown University in 1968, thus justifying this article on pandemics on the smallstatebighistory website. Barry has written a number of other excellent books, including one that showed his Rhode Island roots, a biography of Roger Williams. Indeed, Barry allowed our website to reprint an article he wrote on Roger Williams. (To access this story, go to Archives and type in the search box: barry and “roger williams”).
The 1918 influenza pandemic was the most severe pandemic in modern recent history. It was caused by an H1N1 virus with genes originating in birds. It spread worldwide during 1918-1919.
It is estimated that about 500 million people (about one-third of the world’s then population) became infected with this virus. The number of deaths was estimated to be at least 50 million worldwide (and possibly as much as 100 million) with about 675,000 deaths occurring in the United States.
As usual with severe viruses, mortality was high in people younger than 5 years old and those 65 years and older. What made this virus unusual was the high mortality rate for people aged 20 to 40 years old. The high mortality in healthy people, including those in the 20-40 year age group, was a tragic and unique feature of this pandemic. Perhaps 10% of the young men and women on the planet were killed by the pandemic. Some scientists believe that older people survived at a higher rate because they had built up resistance during the now-forgotten “Russia Flu” of 1898.
No vaccine was ever made to protect against the 1918 influenza infection. And no antibiotics were then available to treat secondary bacterial infections that can be associated with influenza infections. As a result, control efforts worldwide were limited to approaches such as isolation, quarantine, good personal hygiene, use of disinfectants (not ingested in the body), and limitations of public gatherings, which were applied unevenly.
The Great Influenza was popularly known as the Spanish Flu, since the first time it made a public impact was after it broke out in Spain in 1918. But historical studies have shown that it probably began at an overcrowded army post in Kansas in the U.S., at Camp Fuston. It quickly spread to other military camps, as young U.S. soldiers were being trained to be sent over to Europe to fight in World War I in the spring of 1918. To get there, they were crammed onto trains and troop transports for the Atlantic voyage, thus spreading the virus even further.
Local governments in the U.S., not wanting to cause panic, often imposed blackouts on publicizing the virus. A major parade in Philadelphia was allowed to continue, and about 4,600 people died there in a week. By contrast, St. Louis had a lockdown early and suffered comparatively few deaths. A few Midwest towns refused to permit anyone to enter their towns, thus avoiding the pandemic.
The good news is that COVID-19 is not as deadly as the 1918 flu. The bad news is that the coronavirus, which is causing the COVID-19 respiratory illness, appears to be more contagious than the 1918 flu and it takes longer for COVID-19 symptoms to appear –making it easier for the illness to spread.
The remainder of this article is a layman’s summary from a presentation by Dr. Barney S. Graham, Deputy Director of the Vaccine Research Center, National Institute of Allergy and Infectious Diseases, at the National Institutes of Health (NIH), in Bethesda, Maryland.
According to Dr. Graham:
Most experts thought that the next pandemic challenge would be from the influenza virus. With influenza, co-infection or co-influenza viruses get into the same cell in humans or in animals, and an entirely new gene segment can result. When that happens, sometimes a pandemic strain of influenza can arise.
The 1918 influenza looks a lot like what we see today, with auditoriums full of cots because hospital capacity was exceeded, and with masks and distancing entering our everyday lives.

Women ambulance workers practice handling a patient in Washington, D.C. during the 1918 pandemic (CDC)
The 1918 outbreak was particularly challenging because such a high proportion of the earth’s population was exposed. The CDC estimates the death toll at 50 million, but there were excess deaths during the three year period of the outbreak of up to 100 million. It cost up to $5 trillion for the immediate response to the outbreak, which did not include the recovery.
The Great Influenza had a devastating impact on the young and very young, as well as the elderly. Many young people got a syndrome similar to a LRDS Syndrome and died from the influenza. That resulted in a “W” mortality curve and a significant drop in life expectancy in 1918 and 1919. Without the sophisticated medical care we have now, the same kind of result could be potentially happening now. But to be clear, COVID-19 is not as dangerous as these kind of pandemic viral threats.

American soldiers returning from helping to win the war in Europe. They brought the influenza with them to Europe and took it back home with them. The crowded conditions on board the transport vessels led to further spread of the virus (CDC)
In addition, the Great Influenza had a devastating impact on certain populations who were particularly susceptible to this kind of infection. For example, it killed many adults in North American and Eskimo villages, leaving mostly children behind.
Let’s consider current times. The HIV virus showed up in 1981 and the virus was not discovered until 1983. We did not have diagnostic kits until 1984. So compared to COVID-19, when diagnostic kits were available within weeks or months of the outbreak, the situation has improved.
Most emerging infections now are zoonotic (transferred by animals to humans) or vector borne (transferred by the bite of, for example, infected mosquitos, ticks, and fleas). But they can also come from humans.
There have been a number of viruses that have come through over the last few decades, including flaming viruses, Ebola, Zika, Acute Myelitis and the coronaviruses—SARS, MERS and SARS 2 (COVID-19). These viruses are contagious and spread largely through person-to-person contact. Due to airplane travel, a virus can be anywhere on the planet in 24 hours. (McBurney aside: Before the crisis, on the average, about 21,000 Chinese people travelled to the U.S. each day. There were direct flights from Wuhan, China (a city of 11 million people) to San Francisco and New York City.)
We have not made an HIV vaccine that is effective yet. Still, the information we learned working on HIV resulted in our making great strides in developing vaccines for other emerging infection diseases.
Over the last 15 to 20 years, the time for manufacturing a vaccine has dropped down to around 100 days from the time a sequence is found. We believe it may be possible in some cases to have a vaccine product even within 50 days after the first sign of trouble.
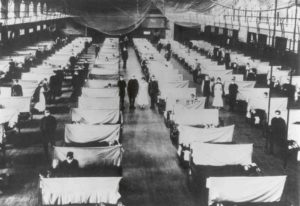
A large hall in Iowa is converted to make room for more hospital beds during the Great Influenza crisis, a scene common today (CDC)
Our experience with RSV (Respiratory Syncytial Virus) changed vaccine development. RSV was recognized as a problem in the 1950s and it continues to be one of the leading causes of infectious disease deaths in infants. Vaccine efforts were first made in in the 1960s. There was no vaccine to prevent RSV, until a recent one showed promise in clinical trials.
[McBurney note: Barney Graham and Peter Kwong of the National Institute of Allergy and Infectious Diseases’ Vaccine Research Center (VRC) at NIH, along with Jason McLellan, a former postdoctoral researcher at VRC and now an associate professor at UT Austin, spearheaded the development of the RSV vaccine candidate DS-Cav1.]Coronaviruses are a large family of viruses. There are hundreds, if not thousands, of coronaviruses hiding in animals, especially in bats.
We have four coronaviruses that routinely circulate in the human population. Those are endemic coronaviruses that cause up to 40% of the common colds in humans.
Over the last 15 or so years, we have had three new beta coronaviruses.
The first was the SARS (Severe Acute Respiratory Syndrome) virus. It appeared in China in November and December of 2002. It was scary and spread throughout most of the world. Then, about eight months later in July, it disappeared and has not come back. The last known transmission was in 2004. SARS had a fatality rate of about 10%.
SARS emerged in November 2002 and it took until March to diagnose it as a virus. Then it took another month to get it sequenced (which is required to start the work for a vaccine). By comparison to what is happening today with COIVD-19, there has been a vast improvement.
But after SARS disappeared, the urgency for a vaccine also disappeared, and researchers stopped working on a vaccine for it. That has hampered efforts dealing with COVID-19 today.
About ten years later, another coronavirus arrived, this one located in the Middle East. Called MERS (Middle East Respiratory Syndrome), it had a much higher fatality rate, almost 40%. But it did not spread beyond the Middle East, except for one big outbreak in South Korea, where about 180 people died.
So with the two beta coronaviruses coming ten years apart, it seemed obvious that it was going to happen again. Researchers starting making vaccine approaches for this prospect focusing on proteins. But because MERS was contained, there was no urgency to follow through on manufacturing a vaccine.
There are more than 100 virus families on earth that have been recognized. Only 25 families have been shown to infect humans. There are vaccine products associated with 13 families. In the other 12 families, there are some viruses that do not have any vaccines available. Within those families, there are a little over 100 other viruses that could potentially be threats from human-to-human contagion.
A real breakthrough occurred when we were able to solve the structure of a spike protein on a human coronavirus. The story behind this is that one of the NIH’s lab workers travelled to the Middle East and came back sick, and his pregnant wife became sick too. But they actually had one of the endemic HPN-1 viruses. It turned out this spike protein was more stable than SARS or MERS so it was easier to work with.
Coronaviruses have been entering the human population for hundreds of years. Two alpha corona viruses are thought to have arrived a few hundred years ago. Many originate in bats or rodents, and then come through some animal intermediate host, such as a camel for MERS or a civet cat for SARS. These events occur every so often. About 20 years ago, what is thought to have been a pandemic occurring in 1881 was discovered.
In the current crisis, China released the sequence of the virus the night of January 10. The next morning the sequences were analyzed, and within a week we were able to make proteins and to get of the structure of the pre-fusion spike using a new cryoelectron microscopy system. So the spike structure was solved quickly, and then the protein was made quickly. Protein is critical because this is the only way the virus can get into human cells. Dr. Graham says (warning, example of technical jargon): “This reception binding domain of one of the protomers and trimer flips up so it can interact with ace 2 molecule that initiates the unfolding of this top part and leave the bottom part as fusion machinery. This is the first step in infection. The way to block that step is antibodies.”
Moderna [a pharmaceutical company NIH is cooperating with to make a vaccine] delivered a GMP (good manufacturing practice) product in record time. This is the first time I have seen the manufacturing process outpace the clinical process and government regulatory process. And even this timeline could have been shortened if we had taken the MERS vaccine all the way through into phase 1 trials. So this three week period could have been shortened even more. By comparison, the fastest previous time line was for Zika. The time frame for GMP today is about 140 days shorter than the Zika effort.
The prototype pathogen approach for pandemic preparedness has been applied to MERS over the last seven years. It was informed by structure-based immunogen-design concepts established for RSV vaccines, and focused on solving coronavirus spike structures, defining mechanisms of CoV neutralization, and evaluating MERS vaccine candidates in collaboration with a commercial mRNA manufacturer (Moderna). Prior spike protein engineering experience resulted in rapid sequence selection and using the mRNA manufacturing platform provided rapid Good Manufacturing Practice (GMP) production of a COVID-19 mRNA vaccine in record time. This candidate was tested in mice in approximately 25 days and humans in approximately 65 days from the time the sequence was released. Clinical and nonclinical evaluation are now proceeding in parallel with hopes to begin efficacy testing before next winter.
[McBurney note: To date, there are now more than 20 efforts worldwide to fast track development of a vaccine, including a collaboration by the University of Oxford and British pharmaceutical company AstraZeneca, a joint venture between Sanofi and GlaxoSmithKline Plc, and a collaboration between a BioNtech SE and American pharmaceutical company Pfizer. Moderna announced around May 1 that it had entered into a pact with a Swisss manufacturer aimed at manufacturing 1 billion doses a year. The proposed vaccine is based on a novel technology that relies on genetic material called mRNA. They expect the first batches to be produced in the U.S. in July.] [Here is some more information I picked up from NIH’s Dr. Anthony Fauci. VRC’s collaboration with Moderna has been a great success so far. It is the fastest time we have ever getting from obtaining the genome sequencing to Phase 1 trials. But having people in Phase 1 trials does not mean we have a vaccine that can be used. It takes another three months to obtain safety and other data. There is still Phase 2. Then it takes time to scale up (manufacture the vaccine). COVID-19 is 75% to 85% analogous to SARS. Before this crisis, widespread lockdowns have never been tried.][Banner image: A barber uses a facemask to shave his customer during the Great Influenza of 1918 (CDC)]
To see a video of Dr. Graham’s presentation, with slides, click on:
https://videocast.nih.gov/summary.asp?live=36377&bhcp=1
The CDC has information on the 1918 pandemic:
https://www.cdc.gov/flu/pandemic-resources/1918-commemoration/1918-pandemic-history.htm